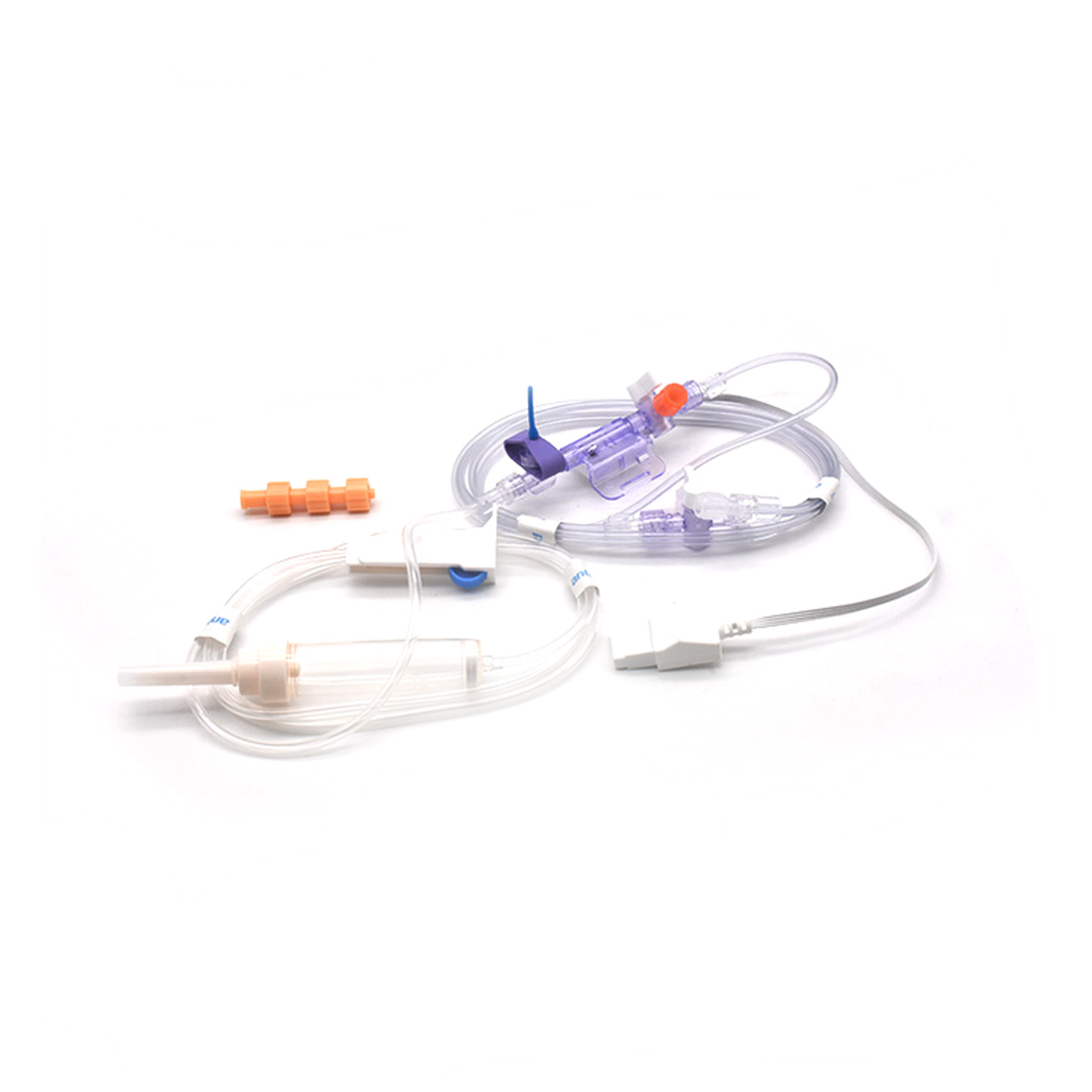
Understanding IBP Transducers and Their Clinical Significance
What Is an IBP Transducer and How Does It Work?
Invasive blood pressure (IBP) transducers are those medical gadgets that take the actual pressure readings from arteries connected via catheters and turn them into electric signals so doctors can watch what's happening in real time. These devices typically have sensors that are kept sterile to pick up even the smallest shifts in pressure levels, then send all that info along to the monitors using systems filled with fluid. The newer models hit around plus or minus 2 mmHg accuracy which really matters when trying to catch dangerous drops or spikes in blood pressure during operations. Research published last year in the Journal of Critical Care Medicine showed something interesting too - patients monitored with these precise transducers had their low blood pressure episodes spotted 42% faster than with regular non-invasive techniques. That kind of early warning makes a big difference in operating rooms where seconds count.
Clinical Importance of Accurate Invasive Blood Pressure Monitoring
For patients needing vasopressors or help stabilizing their circulation, invasive blood pressure (IBP) monitoring is still considered the best approach. Oscillometric methods just don't cut it when we need those beat-by-beat readings that matter so much during septic shock cases, after major accidents, or during heart operations. Doctors depend on how fast these monitors react, typically under one second, to decide about things like giving fluids. We've seen from clinical experience that even waiting five whole minutes to correct blood pressure issues can lead to about 15% higher death rates. A look at ICU practices recently showed something interesting too: hospitals that implemented standard IBP transducer setups saw around 31% fewer mistakes when adjusting medications in their intensive care wards.
CE Certification and EU MDR Compliance for IBP Transducers
What CE Certification Means for Medical Device Safety and Performance
Getting CE certified according to the EU Medical Device Regulation (MDR) basically means an IBP transducer passes tough safety checks needed to sell in Europe. The CE marking shows the product follows important standards like IEC 60601 for medical electronics equipment and also meets those risk management rules from ISO 14971. For devices that comply with MDR regulations, there's this whole process of testing how well materials that touch patients work together safely, making sure they won't cause cell damage or irritation issues. Most blood pressure monitoring devices fall into Class IIa category or above, which means manufacturers can't just declare compliance themselves but need independent experts to verify everything works properly before hitting the market.
Steps to Achieve CE Marking Under EU MDR for IBP Transducers
- Classify the device: Determine risk classification (typically Class IIa for IBP transducers) per MDR Annex VIII.
- Implement a QMS: Establish an ISO 134885-certified quality management system.
- Prepare technical documentation: Compile design specs, risk assessments, and clinical evaluation reports.
- Engage a Notified Body: For Class IIa+ devices, undergo audits of technical files and manufacturing processes.
- Affix CE mark: After conformity assessment, register the device in EUDAMED. Eighty-nine percent of delays stem from incomplete clinical evaluations or outdated QMS documentation, according to SimplerQMS.
Role of Notified Bodies in Validating CE Compliance
The Notified Bodies, or NBs for short, serve as independent auditors when it comes to medical devices classified as higher risk. Their job involves going through all the technical documents and actually showing up at manufacturing sites for inspections. When looking specifically at IBP transducers, these bodies check whether everything lines up with the requirements outlined in MDR Annex II. This includes making sure digital versions have proper software validation and that disposables meet strict sterility standards. According to a recent survey from 2023, most problems found by NBs stem from two main areas: companies not having solid post market surveillance strategies in place, or failing to provide enough biocompatibility data. These findings highlight where manufacturers need to focus their attention if they want to pass audits successfully.
Self-Certification vs. Third-Party Assessment for Class IIa Devices
While Class I non-sterile devices may self-certify, Class IIa IBP transducers require mandatory Notified Body oversight. Self-certification applies only to low-risk accessories such as reusable cables; pressure-sensing components demand full third-party review. Recent MDR updates mandate annual NB audits for Class IIa manufacturers, reducing non-compliant market entries by 34% since 2021.
ISO 10993 Biocompatibility and ISO 14971 Risk Management Standards
Why ISO 10993 Matters for Patient Contact Components in IBP Systems
For IBP transducers that actually touch patients, following ISO 10993 standards is pretty much mandatory if we want to avoid those nasty biological reactions. The whole point of this standard is to figure out what happens when people are exposed for long periods to all sorts of stuff like plastic parts, glue used in assembly, and various sensor bits. According to research published last year, around one in five skin irritation cases reported at hospitals came from medical devices made with materials that didn't meet the ISO 10993-10 requirements. Companies that build these things into their manufacturing process through proper Biological Evaluation Plans tend to run into fewer problems because they test how different materials react when they come into contact with actual bodily fluids and tissues over time.
Evaluating Cytotoxicity, Sensitization, and Irritation Risks
ISO 10993 requires three core tests for IBP transducer materials:
- Cytotoxicity (ISO 10993-5): Measures cell viability after material exposure (’70% cell death permitted)
- Sensitization (ISO 10993-10): Evaluates allergic response potential using guinea pig maximization tests
- Irritation (ISO 10993-10): Assesses localized inflammation risks through epidermal patch testing
Devices failing any test face 89% higher FDA recall rates compared to fully compliant alternatives (Medical Device Reporting, 2022).
Case Study: Material Selection Failures Leading to Non-Compliance
A 2021 EU MDR audit revealed that 32% of rejected IBP transducers used silicone tubing lacking ISO 10993-33 certification for prolonged blood contact. The manufacturer’s $2.7M recall resulted from:
- Assuming "medical-grade" materials automatically met biocompatibility standards
- Skipping accelerated aging tests for chemical leaching risks
- Inadequate documentation of sterilization impacts on material safety
Integrating Biocompatibility Testing with Risk Management per ISO 14971
Combining ISO 10993 data with ISO 14971’s risk framework allows manufacturers to:
- Quantify biological risks using hazard severity/probability matrices
- Implement controls like material substitutions or biocompatible coatings
- Monitor post-market adverse events through ISO 13485-aligned processes
This dual approach reduces late-stage design changes by 41% compared to standalone biocompatibility testing (MedTech Quality Benchmark, 2023).
FDA Clearance and Global Regulatory Requirements for IBP Transducers
FDA 510(k) Clearance Process and Substantial Equivalence
For medical device makers, getting clearance through the FDA's 510(k) program means showing their product is substantially equivalent to something already on the market. About three quarters of all devices got approved this way back in 2023 according to industry figures. Meeting these requirements takes serious testing work and strict adherence to those 21 CFR Part 820 rules about quality systems. Companies that have ISO 13485 certification tend to see their applications processed about 30 percent quicker at the FDA. This seems to come down to better organized paperwork and documentation practices that match what regulators expect to see during review.
Harmonization Challenges: Differences Between EU MDR, FDA, and Other Markets
Divergent regional requirements create compliance complexity:
- EU MDR mandates clinical evaluations for CE marking
- FDA emphasizes predicate-based equivalence
- Japan’s PMDA requires ISO 80369-6 compliance for neuraxial connections
- Brazil’s ANVISA enforces local biocompatibility testing
These discrepancies force manufacturers to maintain 2–3 device variants, increasing development costs by $500k–$1.2M per product (Global MedTech Compliance Report 2024).
Trend: Increasing Stringency in Post-Market Surveillance Requirements
Regulators now demand real-world performance data, with FDA post-market study requirements increasing 40% since 2021. Manufacturers must implement electronic traceability systems to report adverse events within 15 days–50% faster than 2020 timelines. Failure rates for surveillance audits have risen to 22% industry-wide, underscoring the need for proactive risk management (Medical Device Regulatory Journal 2023).
Quality Management and Manufacturing Certifications for IBP Transducer Suppliers
Importance of ISO 13485 in Design and Production of IBP Transducers
The ISO 13485 certification is really important for quality management among IBP transducer suppliers. It helps maintain control across all stages from design through production right down to what happens after products hit the market. What sets this apart from regular quality standards is how it tackles specific needs of medical devices. Think about things like being able to track designs back to their origins, making sure sterilization works properly, and decisions made based on actual risks rather than guesswork. Looking at data from 2023 on recalled medical devices shows something interesting too. Companies with ISO 13485 certification had around half as many problems meeting standards as those without it. When we talk about IBP transducers specifically, following these guidelines means pressure sensors and fluid pathways stay within strict safety margins and perform reliably throughout their entire life cycle in hospitals and clinics.
Good Manufacturing Practice (GMP) Alignment in Contract Manufacturing
Following Good Manufacturing Practice (GMP) guidelines helps contract manufacturers keep things consistent when making IBP transducers that satisfy both FDA and EU MDR standards. What makes GMP so important? Well, it covers three main areas: keeping proper records, making sure equipment stays calibrated, and training staff properly. These aspects have a direct effect on how accurate measurements turn out for those invasive blood pressure monitors. Looking at recent audit data, facilities fully compliant with GMP get around 98% success rate on their first try during sterility tests. That's way better than the roughly 72% seen at places only partly following these rules. When companies outsource parts of the manufacturing process like creating the transducer diaphragms or putting them together, sticking to GMP becomes even more essential. Small mistakes in cleanroom procedures at these third-party sites can really mess up the final product quality.
Frequently Asked Questions (FAQ)
What exactly does an IBP transducer do?
IBP transducers convert pressure readings from arteries into electrical signals, allowing real-time monitoring of blood pressure during critical medical procedures.
Why is CE certification important for IBP transducers?
CE certification ensures that IBP transducers meet strict safety standards required for medical devices sold in Europe, safeguarding patient safety.
How do ISO 10993 standards help in manufacturing IBP transducers?
ISO 10993 standards enable manufacturers to assess materials for biocompatibility, minimizing adverse biological reactions from patient contact.
What role do Notified Bodies play in CE compliance?
Notified Bodies act as independent auditors, validating that IBP transducers meet regulatory requirements before entering the market.
Why is ISO 13485 important for quality management in IBP transducer production?
ISO 13485 ensures that medical device suppliers maintain high standards across design, production, and post-market practices to ensure product quality and safety.
Table of Contents
- Understanding IBP Transducers and Their Clinical Significance
- CE Certification and EU MDR Compliance for IBP Transducers
- ISO 10993 Biocompatibility and ISO 14971 Risk Management Standards
- FDA Clearance and Global Regulatory Requirements for IBP Transducers
- Quality Management and Manufacturing Certifications for IBP Transducer Suppliers
- Frequently Asked Questions (FAQ)